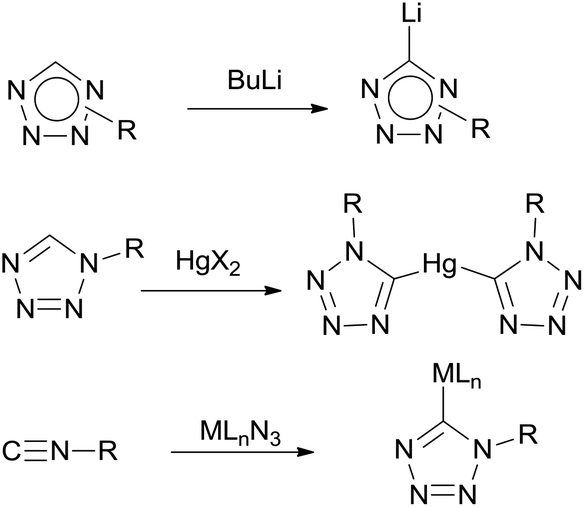
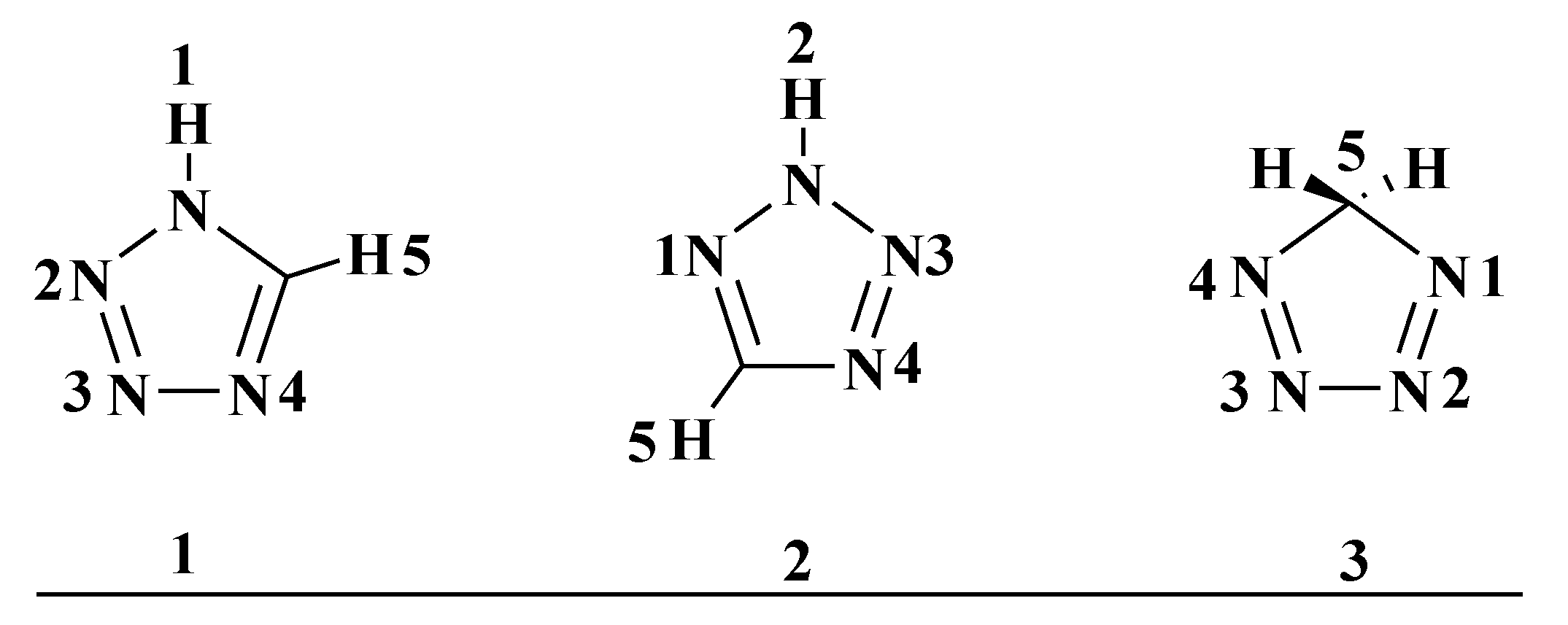
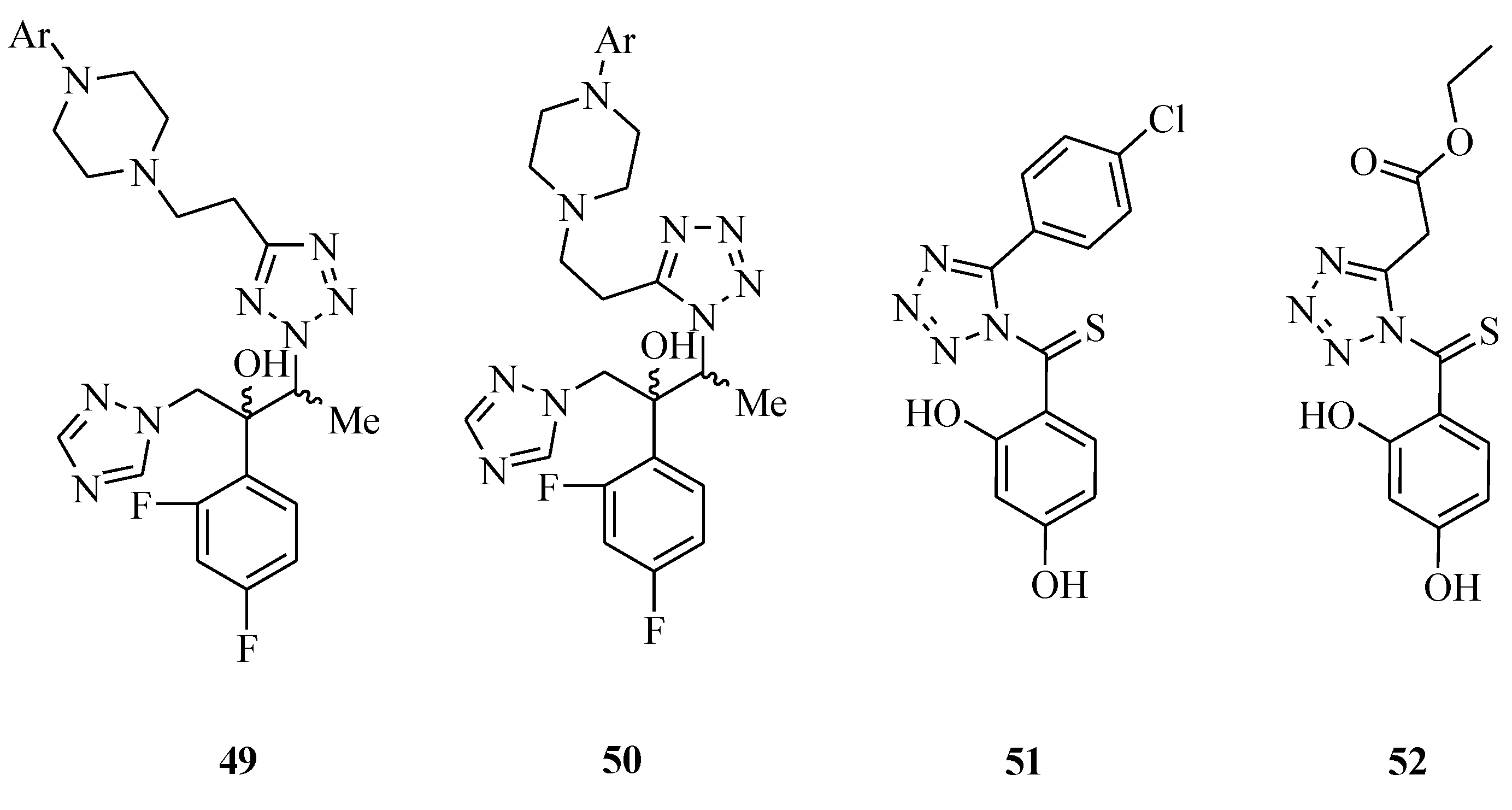
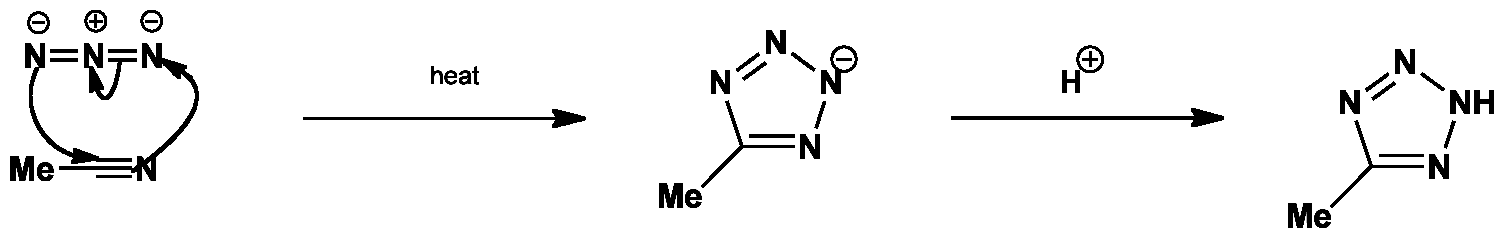
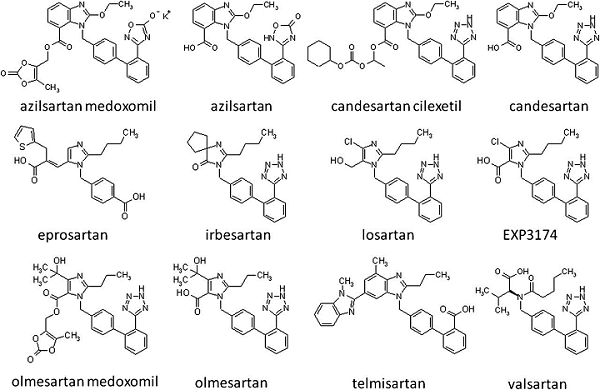
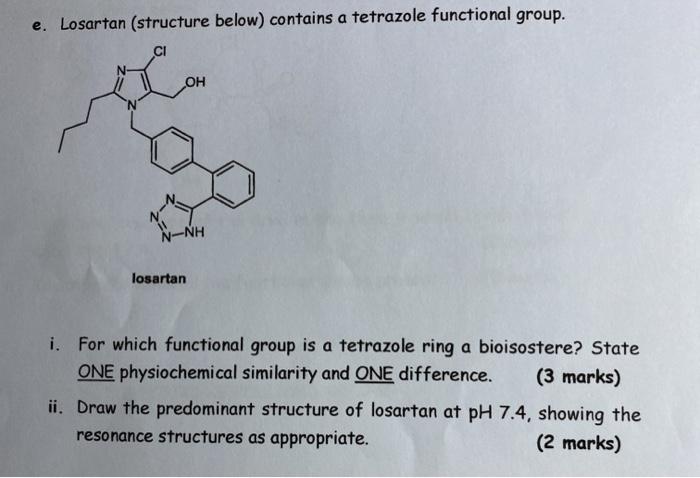
![PDF] Tetrazole compounds: the effect of structure and pH on Caco-2 cell permeability. | Semantic Scholar PDF] Tetrazole compounds: the effect of structure and pH on Caco-2 cell permeability. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/8b95a23e74fb91585f4c34a76b61ef761a84b94a/4-Figure1-1.png)
PDF] Tetrazole compounds: the effect of structure and pH on Caco-2 cell permeability. | Semantic Scholar

EP2748157B1 - Method for producing tetrazole-substituted anthranilic acid diamide derivatives by reacting benzoxazinones with amines - Google Patents

Ring Opening of Tetrazole via Unusual Vilsmeir-Haack Reaction Forming Novel Triazenes | Bentham Science

Asymmetric synthesis of tetrazole and dihydroisoquinoline derivatives by isocyanide-based multicomponent reactions | Nature Communications
PROCESS FOR THE PREPARATION OF TETRAZOLE DERIVATIVES FROM ORGANO BORON AND ORGANO ALUMINIUM AZIDES - Patent 1646636

The structure of cefazolin drug containing 1-substituted tetrazole ring. | Download Scientific Diagram
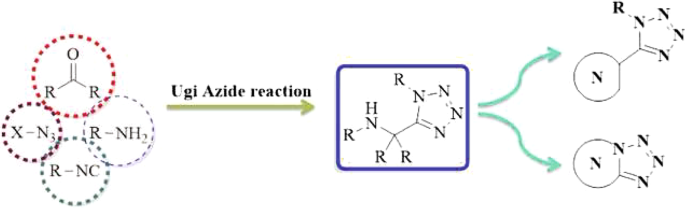
Synthesis of N-heterocycles containing 1,5-disubstituted-1H-tetrazole via post-Ugi-azide reaction | SpringerLink