Revealing sodium-ion diffusion in alluaudite-type Na4–2xM1+x(MoO4)3 (M = Mg, Zn, Cd) from 23Na MAS NMR and ab initio studies - ScienceDirect

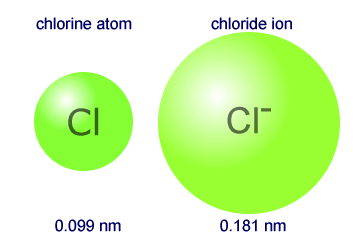
6.3 Periodic Trends Sodium chloride (table salt) produced the geometric pattern in the photograph. Such a pattern can be used to calculate the position. - ppt video online download
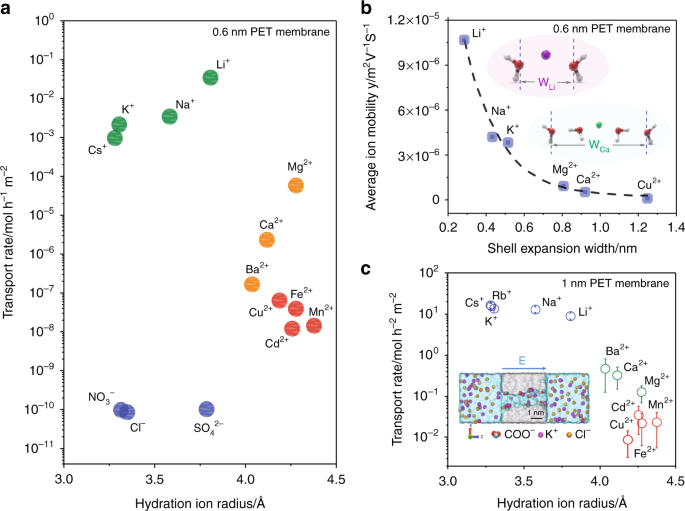
Design principles of ion selective nanostructured membranes for the extraction of lithium ions | Nature Communications

Hard Carbon as Sodium‐Ion Battery Anodes: Progress and Challenges - Xiao - 2019 - ChemSusChem - Wiley Online Library

Recent Progress in Rechargeable Sodium‐Ion Batteries: toward High‐Power Applications - Pu - 2019 - Small - Wiley Online Library
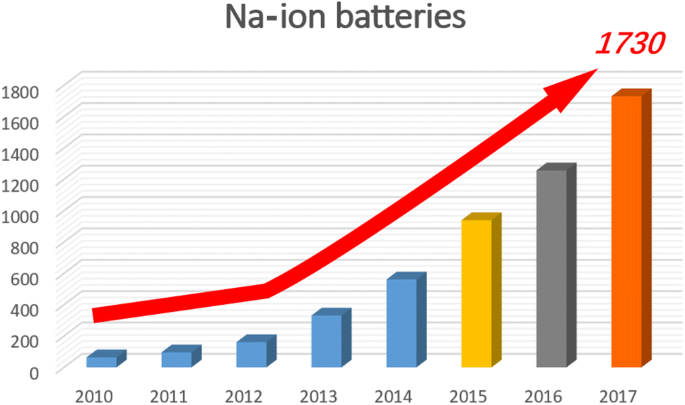
Electrode Materials for Sodium-Ion Batteries: Considerations on Crystal Structures and Sodium Storage Mechanisms | SpringerLink

The relationship between the size of the ions in the ionic liquid and... | Download Scientific Diagram

Iron substitution in Na4VMn(PO4)3 as a strategy for improving the electrochemical performance of sodium-ion batteries - ScienceDirect

Revealing sodium-ion diffusion in alluaudite-type Na4–2xM1+x(MoO4)3 (M = Mg, Zn, Cd) from 23Na MAS NMR and ab initio studies - ScienceDirect